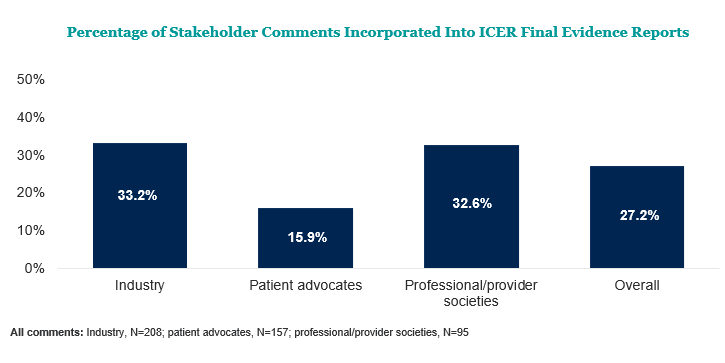
To examine this issue more closely, one aspect of the ICER stakeholder engagement process– the public comment period — was considered. This analysis, conducted by Xcenda, analyzed four of ICER’s final value assessment reports (non-small cell lung cancer, osteoporosis’, ovarian cancer, and migraine) to determine the extent to which ICER has acknowledged and incorporated input from patients and other stakeholders.
Key Findings
- Since refining its process for public commenting in 2017, ICER has acknowledged more than 95% of comments received from stakeholders.
- However, only 27% of all stakeholder comments were incorporated into ICER’s final assessments. Comments from patient advocates were half as likely to be incorporated compared to other stakeholders.
- Even when stakeholders provided proposed solutions to address their comments, ICER incorporated only one third of such comments.
- Patient advocates were most likely to comment on the adequacy of existing evidence (10.2%), the incorporation of the patient perspective (8.3%), and transparency (7.6%).
Where We Stand on Value Assessment
Process
- Develop a transparent process through which frameworks are developed, implemented and validated. Value frameworks and assessments should be transparent and available to patients to fully understand the assumptions that serve as their foundation. Value framework developers should also be transparent about the feedback they receive from patients and other stakeholders, noting where the framework incorporates the feedback received or their rationale for doing or not doing so.
- Meaningfully engage with patient and provider organizations. Value framework developers should engage with organizations representing the impacted patient communities and clinical experts in the specific treatment area under consideration. Meaningful engagement should occur in advance of scoping assessments, as well as throughout the process, to ensure that assessments are achieving consensus on the assumptions, definitions and underlying questions.
Standards
- Rely on a range of sound, patient-centered sources of evidence. Value assessment frameworks should rely on high-quality, rigorously developed methods of evidence that fully capture value that matters to patients. Value frameworks should also be completely transparent about the evidence on which the assessments are based, as well as the limitations of the evidence.
- Reflect patient-centered outcomes. Quantifying value in a way that is useful and meaningful to patients and people with disabilities requires a basic understanding of their values and preferences. Doing so will benefit both patient and payer as they identify and integrate the appropriate patient-centered criteria in assessing the value of treatments for a particular condition.
- Address costs holistically, including costs that matter to patients. Value frameworks and assessments should have a holistic perspective on the economic component of value, and include long-term, personal and societal costs, such as risk of disability and the potential need for caregiving. Broader costs should be considered rather than focusing only on short-term costs.
- Support shared decision-making. Focus efforts on advancing shared decision-making between patients and physicians. Well-informed decisions by patients and consumers are foundational to advancing value in ways that are patient-centered.
Safeguards:
- Acknowledge diversity and differences among patients, and avoid the “one-size-fits-all” mentality of value. Value frameworks should ensure that their models and methods account for important differences in patient preferences, characteristics and treatment goals.
- Preserve protections in the Affordable Care Act against use of comparative or cost effectiveness research to make centralized value judgements. Value frameworks have the potential to support informed treatment decision-making between patients and their physicians. However, if used in appropriately to make centralized value judgements, value assessments could also limit patient access to necessary medical treatment
| pipc_one_pager_on_xcenda.pdf |
| pipc_xcenda_icer_stakeholder_mapping_final_report_2018___003_.pdf |
