PIPC Submits Comments to PCORI on Proposed Research AgendaIn a letter to the Patient-Centered Outcomes Research Institute (PCORI), PIPC offered feedback on PCORI's proposed research agenda. In the letter, PIPC Chair Tony Coelho doubled down on his recommendation that PCORI engage in direct outreach to organizations representing patients and people with disabilities in its forthcoming research efforts, noting that PCORI must be more specific and clear with respect to its methodologies. The letter also stresses that PCORI should work with experts that do not view the discriminatory quality-adjusted-life-years (QALY) metric as the "gold standard" for value assessment in health care.
|
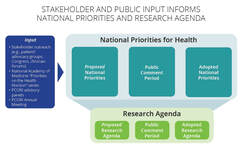
PIPC Offers Comments on PCORI National Priorities for HealthOn August 27, 2021, PIPC submitted a comment letter on PCORI's Proposed National Priorities for Health. The letter encourages PCORI work closely with stakeholders in identifying the topics to be prioritized within each broad area, the research agenda for each topic, and the outcomes data to be collected and analyzed as part of the research. PIPC also reiterated its strong support for PCORI’s mandate of conducting research on the comparative clinical effectiveness of medical treatments and services, as well as the statutory prohibition against cost- effectiveness analysis. In a comment letter to the Patient-Centered Outcomes Research Institute (PCORI), the Partnership to Improve Patient Care (PIPC) offered feedback on PCORI's Proposed National Priorities for Health.
|
40 Leading Organizations Join PIPC Letter on PCORI Outcome PrinciplesOver 40 leading groups and individuals representing the patient and disability communities signed onto the Partnership to Improve Patient Care's (PIPC) comment letter to the Patient-Centered Outcomes Research Institute (PCORI) on its Proposed Principles for the Consideration of the Full Range of Outcomes Data. The letter applauds Executive Director Nakela Cook for recognizing the PCORI authorizing statue's prohibition on cost-effective analysis, thus protecting patients from harmful and restrictive coverage decisions. The letter also emphasizes three key recommendations to PCORI, including: (1) promote usability of collected information for decision-making, including patients with multiple chronic conditions and their caregivers; (2) contextualize the cost information being communicated to ensure it is not used against patients and providers; and (3) solicit and appoint new Methodology Committee members who have appropriate expertise in the collection and communication of patient-level data.
|
Targeted Literature Review on Patient-Centered Cost OutcomesIn September 2020, the Board of Governors for the Patient-Centered Outcomes Research Institute (PCORI) approved the proposed Principles for the Consideration of the Full Range of Outcomes Data for public comment. The goal of these principles is to outline PCORI’s compliance with its reauthorization legislation, which states that, in addition to clinical outcomes, research should also endeavor to capture patient-important outcomes that assess the economic burden of treatments and services. Click here to view PIPC's white paper
|
PCORI and ICER Background, Funding, and Impact on Patient-Centered CareAmidst the debate around value driven health care, it is important to understand what organizations can support such a system. Two prominent organizations, the Patient-Centered Outcomes Research Institute (PCORI) and the Institute for Clinical and Economic Review (ICER), do work to help decision makers compare available treatment options. However, their approaches differ significantly. Click here to view PIPC's primer on this issue.
|
In Letter to PCORI, PIPC Offers Input on Next 10 YearsIn a 2020 letter to the Patient-Centered Outcomes Research Institute (PCORI), Partnership to Improve Patient Care (PIPC) Chairman Tony Coelho offered input on the next 10 years of PCORI. Chairman Coelho framed PIPC’s recommendations around three core principles: relevance, timeliness, and trust. Specifically, he encouraged PCORI to: (1) create a national agenda for research priorities; (2) expand collection of and access to patient-centered outcomes; (3) advance use of patient-centered outcomes in value assessment; and (4) promote patient-centered methodologies. “This is a crucial moment to build on PCORI’s success for another 10 years,” wrote Chairman Coelho. “Success, we believe, will entail effectively connecting PCORI and its research strengths to the current, pressing needs of our health care system and the patients and caregivers it serves.
|
Congress Reauthorizes PCORI for 10 YearsPIPC and members of Friends of PCORI Reauthorization applauded Congress for extending funding for the Patient-Centered Outcomes Research Institute (PCORI) for an additional ten years. PIPC Chairman and Friends of PCORI Reauthorization co-chair Tony Coelho stated, "I am pleased that Congress responded to the strong support of 200 plus stakeholder organizations for a long-term reauthorization of PCORI’s patient-centered mission. The next 10 years of PCORI is an opportunity to drive an efficient and informed health system that is truly patient-centered and responsive to the individual characteristics, needs and priorities of patients and people with disabilities." Click here to view the full press release.
|
Driving Accountability for Value in Medicine: Leveraging PCORI through ReauthorizationPatients and persons with disabilities are increasingly concerned about whether they can afford the tests and treatments they need. As stated by the Partnership to Improve Patient Care (PIPC) in 2010, comparative effectiveness research can form the foundation for meeting “the critically important challenge of controlling health care costs while avoiding oversimplified rationing of patient care.” Now more than ever, we need solutions that are both evidence-based and patient-centered.
The Patient-Centered Outcomes Research Institute (PCORI) offers important infrastructure to meet this challenge. With key reforms included with its FY2019 reauthorization, PCORI’s work can be focused to ensure its research is timely and responsive to the needs of those making decisions about new drugs and other treatment options, and that its research findings are more readily available to decision-makers. |
| pcori_solution_3__002_.pdf.pdf | |
| File Size: | 99 kb |
| File Type: | |
Primer: PCORI Background, Funding Streams, and Reauthorization The Patient-Centered Outcomes Research Institute (PCORI) was created in 2010 to establish priorities and set an agenda for the conduct of comparative clinical effectiveness research (CER). The Government Accountability Office (GAO) concluded in a March 2015 report that PCORI is fulfilling its Congressional mandate to generate evidence that patients and those who care for them can use to make better-informed healthcare decisions. PCORI invested nearly $1.4 billion in more than 500 patient-centered CER studies and related projects that support CER to date.
Click here to read more about PCORI, including why the institute matters, how it's funded, and reauthorization in 2019. For more information and fact sheets about PCORI-funded research, click here. |
Principles of Patient-Centeredness in ResearchIn order to put patients and providers first, comparative clinical effectiveness research must:
|
PCORI Contracts by StateTo learn more about PCORI's work, the research they fund, and their programs and initiatives, click here.
|
PIPC and Families USA RoundtableOn June 19, 2014, PIPC and Families USA co-hosted a roundtable discussion on “Accountability for Patient Engagement in Research and Dissemination.” The purpose was to move beyond a discussion of the points of engagement, and focus on who is accountable for patient engagement, and what makes engagement meaningful. It was noted, and agreed by participants, that a goal of patient engagement is real patient empowerment and activation in their health care. A focus of the discussion was implementation of the Patient-Centered Outcomes Research Institute (PCORI), which is creating a precedent for patient engagement practices in research that could be modeled by other entities.
|